
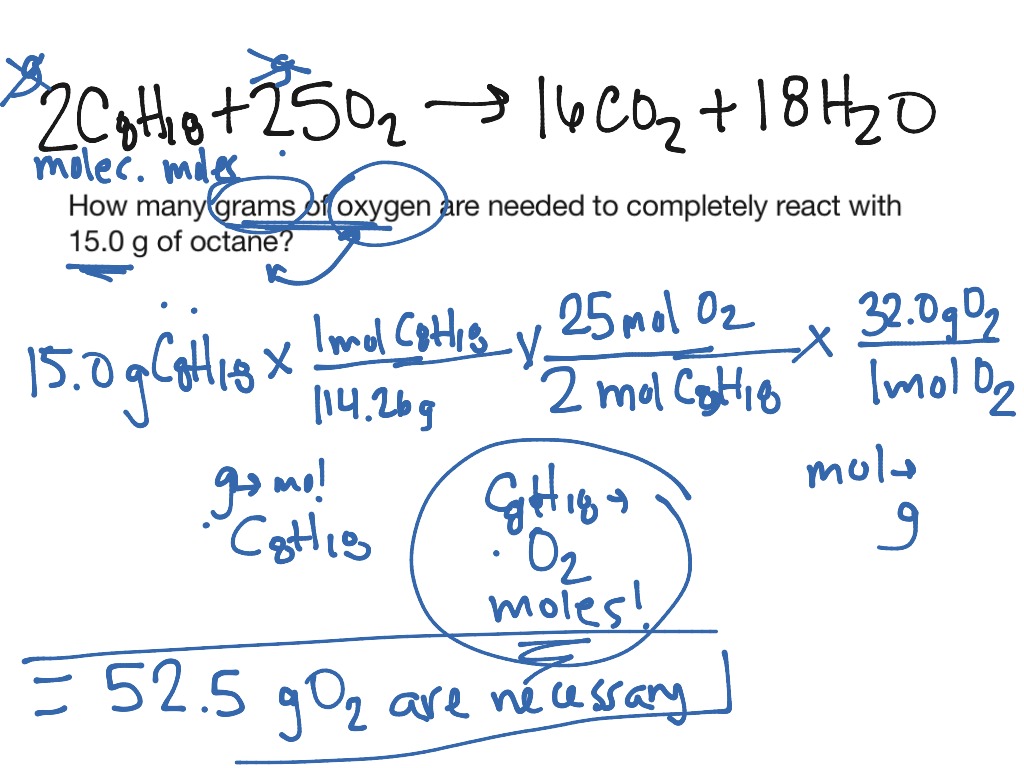
Remember they are only guidelines to help if you run into trouble. If you can see the balanced equation by sight, you don't need to go by the guidelines. This is a perfectly acceptable balanced chemical equation.Īt the very beginning of this problem, perhaps you could see this was the answer. We can get rid of the fractional coefficients by multiplying by 2 even though
Is not explicitly included when writing the chemical equation. Note: Typically a stoichiometric coefficient of "1" This we will need to use fractional coefficients. Now we can balance the remaining single-element compounds. Assign a stoichiometricĬoefficient of 1 to the most complex compound, NO. This equation is not balanced since there are more N and O atoms on the left Therefore, sometimes it may be necessary to deviate from these general

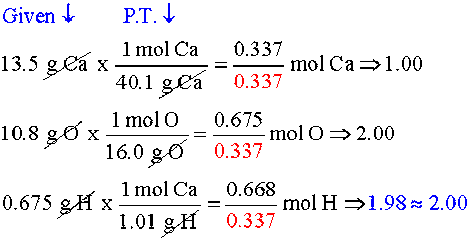
In chemistry it is very important to understand the relationship between reactantsĪnd products in a reaction. Overview: In this tutorial, the fundamentals of balancing chemical


 0 kommentar(er)
0 kommentar(er)
